DNA replication is a critical biological process that cells use to copy their genome – but when it goes wrong, those failures can have dire consequences. Missteps in the replication process are key drivers of cancer, contributing to genetic mutations, tumor progression, and the development of resistance to treatment. Yet understanding how and why these problems occur is a longstanding challenge.
Now, ASPIRE award recipients Bennie Lemmens, PhD, and Jiri Bartek, MD, PhD, both of Karolinska Institutet and SciLifeLab in Stockholm, Sweden, have developed innovative technologies that provide unprecedented control and visualization of human DNA replication. These findings, published in two recent studies in the EMBO Journal and Nature Communications, open up promising new avenues for improving cancer therapy design and precision diagnostics.
Mapping Replication Nanostructures (The EMBO Journal)
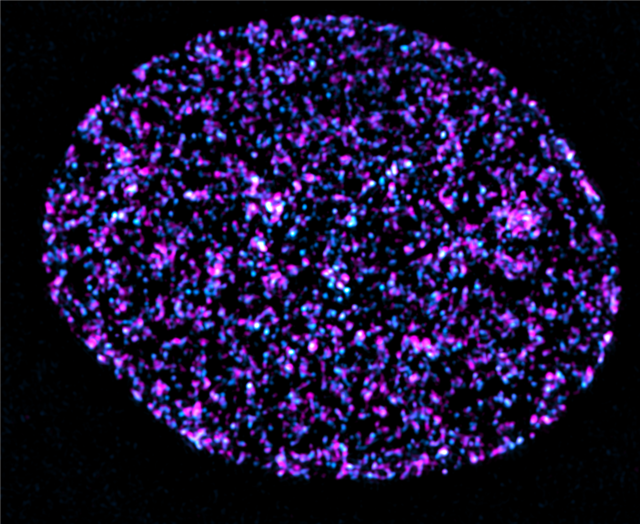
The EMBO Journal publication unveils a suite of powerful approaches for visualizing DNA replication nanostructures, some of the most minute components of human cells. Their framework, 3D-SPARK, utilizes optimized nucleotide labeling strategies in conjunction with super-resolution microscopy (which employs advanced technology to overcome the resolution limitations of conventional light microscopes) to detect and classify nanoscale DNA synthesis events with unprecedented precision in both space and time.
To validate these findings, the team also developed XMARK, a method that uses expansion microscopy to physically enlarge cellular structures, enabling detailed imaging of replication sites with standard microscopes.
By integrating these two technologies, the team showed how replication nanostructures reorganize in response to cancer-causing gene activation, chemotherapy drugs, and the disruption of the nucleus. This approach offers a versatile platform for scientists to quantify how DNA replication is altered in both healthy and diseased cells.
Controlling DNA Replication Licensing (Nature Communications)
In a separate study in Nature Communications, the researchers identified a key regulator of DNA replication licensing. This licensing process is the crucial first step of DNA replication, where a cell gets “permission” to begin copying its DNA.
Using a combination of chemical genetics (using small molecules to control protein function) and advanced sequencing, the team demonstrated that the CDK4/6 protein actively promotes this licensing step in human cells. This finding challenges a long-standing model from yeast studies where similar proteins were thought to inhibit the process.
The study also shows that CDK4/6 inhibitors, a mainstay of breast cancer treatment, work by preventing replication licensing. The authors showed that using these drugs in specific combinations can force cancer cells with defects in the p53 tumor-suppressor gene into making lethal errors during their cell cycle. These insights change the current understanding of how cells control replication and highlight new therapeutic opportunities for cancers driven by unregulated cell growth.
Implications for Cancer Research and Therapy
Together, these breakthroughs in both visualizing and controlling DNA replication provide a powerful new framework for cancer research. Understanding the physical machinery of replication in high definition, combined with knowing how to manipulate its key regulators, will allow researchers to pinpoint and exploit new vulnerabilities unique to cancer cells.
“This dual achievement exemplifies the spirit of the ASPIRE award—pursuing bold, high-risk, high-reward research that bridges molecular cell biology, chemical biology, and cancer therapy,” said Ryan Schoenfeld, PhD, CEO of The Mark Foundation for Cancer Research. “The ability to precisely map replication and understand its druggable checkpoints will inform both predictive biomarkers and next-generation therapeutic strategies.”



